Blogs
Pharmacogenomics: Key to Unlock Mystery Behind Statin Response
Cardiovascular Diseases (CVD) are a group of disorders that encompass coronary heart disease, stroke and ischemic attack, peripheral arterial disease and aortic disease. They are the leading cause of premature death worldwide and one of the major causes of the increasing healthcare burden on both the patients and country. Risk factors for CVDs include high blood pressure, smoking, high cholesterol, diabetes, sedentary lifestyle, obesity, family history of CVD, ethnicity, etc. In addition to an active lifestyle and healthy food habits, medications are often prescribed to bring some of the erratic biomarkers like cholesterol under control.
Statins are a group of frequently prescribed class of medicines for CVD that helps to reduce the low-density lipoprotein (LDL) or “bad cholesterol” in the blood by inhibiting an enzyme called 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (HMGCR). This enzyme is responsible for the formation of LDL in the liver, increase in the activity of surface level receptor for LDL and increased uptake of LDL by liver. Use of statins has been consistently associated with reduction in the risk for CVDs.
Adverse drug response with respect to statin use has been time and again reported and well documented. Over 100 million patients are estimated to be prescribed statins every year. Statin-induced myopathy is well known, and includes clinical manifestations such as rhabdomyolysis, myalgia and myositis, and is characterized by a mild to severe form of muscle weakness and elevated levels of creatine kinase, a blood marker for muscle cell damage. Most statin-associated muscle symptoms have been observed within six months of statin medication in most patients and tend to disappear upon lowering of dose or discontinuation.
Increased risk for statin-induced myopathy has been observed in females and those with diabetes, untreated hypothyroidism, high blood pressure, liver and kidney diseases, high alcohol consumption, etc. In addition, genetic factors have also been shown to contribute to adverse drug response to statins.
Pharmacogenomics is the integration of pharmacology and genomics which explores the role of genetic makeup of an individual’s response to drugs. The genetic sequence of one person differs from another due to the presence of variations throughout the genome. The association of genetic variations with statin response has been studied by researchers both single gene association and genome wide association. Both type of studies have been conducted in genes that modulate statin pharmacokinetics (transport and metabolism), efficacy and organ toxicity. While genes involved in statin pharmacokinetics and organ toxicity have been shown to determine onset and severity of statin-induced adverse effects, variations that moderate efficacy are thought to indirectly effect toxicity.
A comprehensive analysis of 9 different studies on 4442 patients showed that individuals with a specific genetic variation (T>C variation, where T is the sequence found in majority of population, also known as major allele and C is the sequence found in smaller percentage of the population, also known as minor allele) in SLCO1B1 gene were more likely to develop statin-related myopathy, particularly for simvastatin.
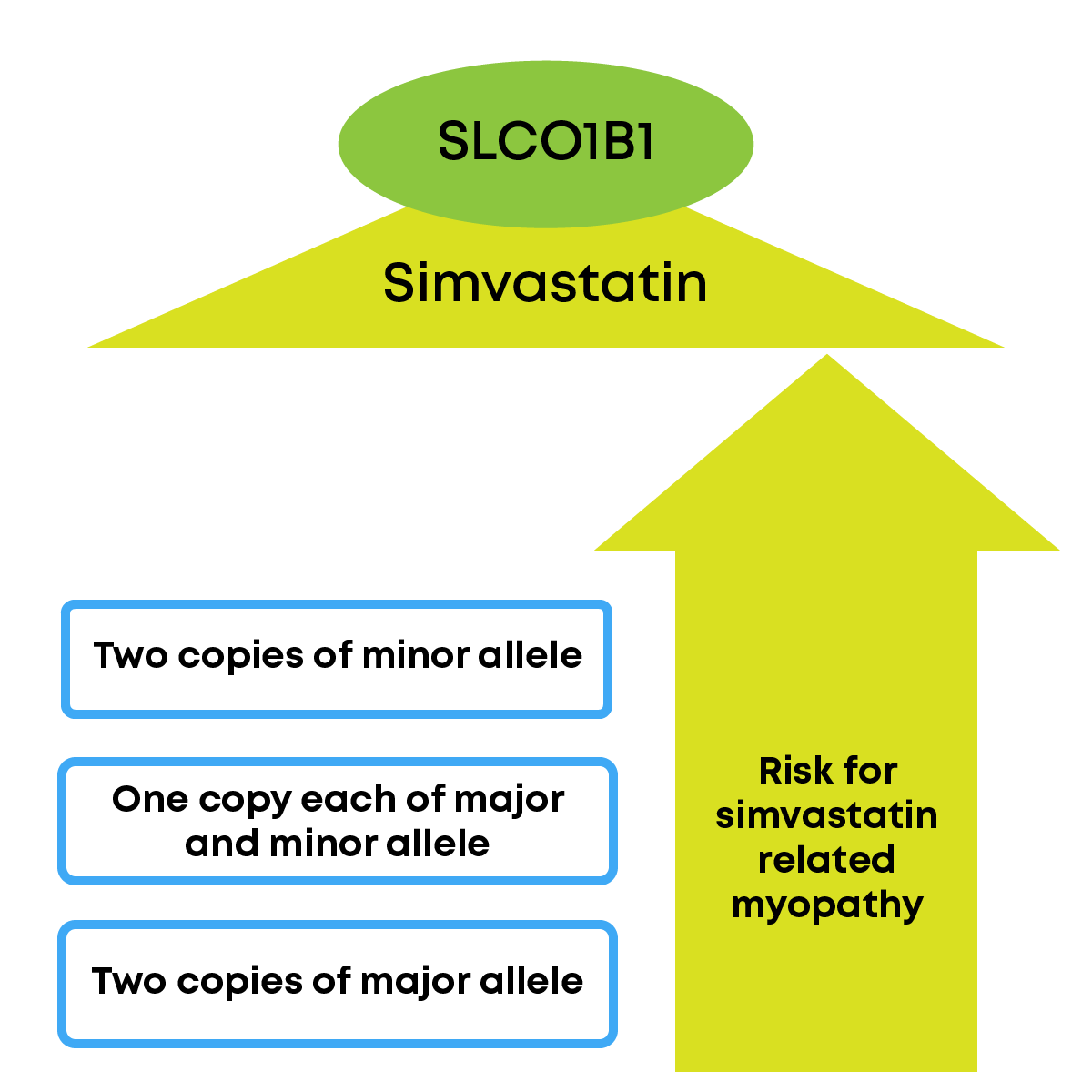
The Clinical Pharmacogenetics Implementation Consortium (CPIC) has made dosage recommendation of drug simvastatin based on the presence of the variant in patients. Pharmacogenetic studies on gene-statin interaction in the Indian context, unfortunately, are sparse.
Curegen is a pharmacogenomics test from MedGenome Labs Ltd. A total of 723 variations across 18 genes effecting medicines prescribed for cardiovascular diseases, cancers, psychiatric disorders, transplant, pulmonary diseases, infections/HIV, etc. Basic and advanced testing panels would be available depending upon number of genes/variations required to be screened. All tests offered are based on CPIC guidelines, and only those with significant level of evidence are reported.
With the help of inputs from a pharmacogenetic test such as CUREGEN, a clinician will be able to:
Collective application of information from a pharmacogenomic test on statin use can pave way for effective therapeutic suggestion through dose modification or alternative statin. It can also help to avoid discontinuation of statin (improve medication compliance) and facilitate patients in getting continuous medical care for their cardiovascular condition.
References Alzghari S K (April 30, 2018) An Unnecessary Pain: Using Pharmacogenetics for Statin-related Skeletal Muscle Toxicity. Cureus 10(4): e2557. DOI 10.7759/cureus.2557
Kitzmiller JP, Mikulik EB, Dauki AM, Murkherjee C, Luzum JA. Pharmacogenomics of statins: understanding susceptibility to adverse effects. Pharmgenomics Pers Med. 2016 Oct 3;9:97-106. doi: 10.2147/PGPM.S86013. PMID: 27757045; PMCID: PMC5055044.
Reach out to us at: www.genessense.com | youfirst@genessense.com | 1800 296 9696
Recent Blogs